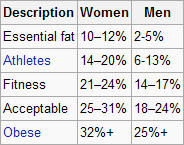Body fat is not an inert deposit of energy. It can be seen as a distributed endocrine organ. Body fat cells, or adipocytes, secrete a number of different hormones into the bloodstream. Major hormones secreted by adipose tissue are adiponectin and leptin.
Estrogen is also secreted by body fat, which is one of the reasons why obesity is associated with infertility. (Yes, abnormally high levels of estrogen can reduce fertility in both men and women.) Moreover, body fat secretes tumor necrosis factor-alpha, a hormone that is associated with generalized inflammation and a number of diseases, including cancer, when in excess.
The reduction in circulating tumor necrosis factor-alpha and other pro-inflammatory hormones as one loses weight is one reason why non-obese people usually experience fewer illness symptoms than those who are obese in any given year, other things being equal. For example, the non-obese will have fewer illness episodes that require full rest during the flu season. In those who are obese, the inflammatory response accompanying an illness (which is necessary for recovery) will often be exaggerated.
The exaggerated inflammatory response to illness often seen in the obese is one indication that obesity in an unnatural state for humans. It is reasonable to assume that it was non-adaptive for our Paleolithic ancestors to be unable to perform daily activities because of an illness. The adaptive response would be physical discomfort, but not to the extent that one would require full rest for a few days to fully recover.
Inflammation markers such as C-reactive protein are positively correlated with body fat. As body fat increases, so does inflammation throughout the body. Lipid metabolism is negatively affected by excessive body fat, and so is glucose metabolism. Obesity is associated with leptin and insulin resistance, which are precursors of diabetes type 2.
Some body fat is necessary for survival; that is normally called essential body fat. The table below (from Wikipedia) shows various levels of body fat, including essential levels. Also shown are body fat levels found in athletes, as well as fit, “not so fit” (indicated as "Acceptable"), and obese individuals. Women normally have higher healthy levels of body fat than men.
If one is obese, losing body fat becomes a very high priority for health reasons.
There are many ways in which body fat can be measured.
When one loses body fat through fasting, the number of adipocytes is not actually reduced. It is the amount of fat stored in adipocytes that is reduced.
How much body fat can a person lose in one day?
Let us consider a man, John, whose weight is 170 lbs (77 kg), and whose body fat percentage is 30 percent. John carries around 51 lbs (23 kg) of body fat. Standing up is, for John, a form of resistance exercise. So is climbing stairs.
During a 24-hour fast, John’s basal metabolic rate is estimated at about 2,550 kcal/day. This is the number of calories John would spend doing nothing the whole day. It can vary a lot for different individuals; here it is calculated as 15 times John’s weight in lbs.
The 2,550 kcal/day is likely an overestimation for John, because the body adjusts its metabolic rate downwards during a fast, leading to fewer calories being burned.
Typically women have lower basal metabolic rates than men of equal weight.
For the sake of discussion, we expect each gram of John’s body fat to contribute about 8 kcals of energy, assuming a rate of conversion of body fat to calories of about 90 percent.
Thus during a 24-hour fast John burns about 318 g of fat, or about 0.7 lbs. In reality, the actual amount may be lower (e.g., 0.35 lbs), because of the body's own down-regulation of its basal metabolic rate during a fast. This down-regulation varies widely across different individuals, and is generally small.
Many people think that this is not much for the effort. The reality is that body fat loss is a long term game, and cannot be achieved through fasting alone; this is a discussion for another post.
It is worth noting that intermittent fasting (e.g., one 24-hour fast per week) has many other health benefits, even if no overall calorie restriction occurs. That is, intermittent fasting is associated with health benefits even if one fasts every other day, and eats twice one's normal intake on the non-fasting days.
Some of the calories being burned during John's 24-hour fast will be from glucose, mostly from John’s glycogen reserves in the liver if he is at rest. Muscle glycogen stores, which store more glucose substrate (i.e., material for production of glucose) than liver glycogen, are mobilized primarily through anaerobic exercise.
Very few muscle-derived calories end up being used through the protein and glycogen breakdown pathways in a 24-hour fast. John’s liver glycogen reserves, plus the body’s own self-regulation, will largely spare muscle tissue.
The idea that one has to eat every few hours to avoid losing muscle tissue is complete nonsense. Muscle buildup and loss happen all the time through amino acid turnover.
Net muscle gain occurs when the balance is tipped in favor of buildup, to which resistance exercise and the right hormonal balance (including elevated levels of insulin) contribute.
One of the best ways to lose muscle tissue is lack of use. If John's arm were immobilized in a cast, he would lose muscle tissue in that arm even if he ate every 30 minutes.
Longer fasts (e.g., lasting multiple days, with only water being consumed) will invariably lead to some (possibly significant) muscle breakdown, as muscle is the main store of glucose-generating substrate in the human body.
In a 24-hour fast (a relatively short fast), the body will adjust its metabolism so that most of its energy needs are met by fat and related byproducts. This includes ketones, which are produced by the liver based on dietary and body fat.
How come some people can easily lose 2 or 3 pounds of weight in one day?
Well, it is not body fat that is being lost, or muscle. It is water, which may account for as much as 75 percent of one’s body weight.
References:
Elliott, W.H., & Elliott, D.C. (2009). Biochemistry and molecular biology. New York: NY: Oxford University Press.
Fleck, S.J., & Kraemer, W.J. (2004). Designing resistance training programs. Champaign, IL: Human Kinetics.
Large, V., Peroni, O., Letexier, D., Ray, H., & Beylot, M. (2004). Metabolism of lipids in human white adipocyte. Diabetes & Metabolism, 30(4), 294-309.
Sunday, February 28, 2010
Thursday, February 25, 2010
Corn Oil and Cancer?
The benefits of corn oil keep rolling in. In a new study by Stephen Freedland's group at Duke, feeding mice a diet rich in butter and lard didn't promote the growth of transplanted human prostate cancer cells any more than a low-fat diet (1).
Why do we care? Because other studies, including one from the same investigators, show that corn oil and other industrial seed oils strongly promote prostate cancer cell growth and increase mortality in similar models (2, 3).
From the discussion section:
* The average American eats 7-8% omega-6 by calories. This means it will be difficult to see a relationship between omega-6 intake and cancer (or heart disease, or most things) in observational studies in the US or other industrial nations, because we virtually all eat more than 4% of calories as omega-6. Until the 20th century, omega-6 intake was below 4%, and usually closer to 2%, in some traditional societies. That's where it remains in contemporary traditional societies unaffected by industrial food habits, such as Kitava.
Why do we care? Because other studies, including one from the same investigators, show that corn oil and other industrial seed oils strongly promote prostate cancer cell growth and increase mortality in similar models (2, 3).
From the discussion section:
Current results combined with our prior results suggest that lowering the fat content of a primarily saturated fat diet offers little survival benefit in an intact or castrated LAPC-4 xenograft model. In contrast to the findings when omega-6 fats are used, these results raise the possibility that fat type may be as important as fat amount or perhaps even more important.There's a large body of evidence implicating excess omega-6 fat in a number of cancer models. Reducing omega-6 to below 4% of calories has a dramatic effect on cancer incidence and progression*. In fact, there have even been several experiments showing that butter and other animal fats promote cancer growth to a lesser degree than margarine and omega-6-rich seed oils. I discussed that here.
* The average American eats 7-8% omega-6 by calories. This means it will be difficult to see a relationship between omega-6 intake and cancer (or heart disease, or most things) in observational studies in the US or other industrial nations, because we virtually all eat more than 4% of calories as omega-6. Until the 20th century, omega-6 intake was below 4%, and usually closer to 2%, in some traditional societies. That's where it remains in contemporary traditional societies unaffected by industrial food habits, such as Kitava.
Monday, February 22, 2010
Magnesium and Insulin Sensitivity
From a paper based on US NHANES nutrition and health survey data (1):
Magnesium status is associated with insulin sensitivity (2, 3), and a low magnesium intake predicts the development of type II diabetes in most studies (4, 5) but not all (6). Magnesium supplements largely prevent diabetes in a rat model* (7). Interestingly, excess blood glucose and insulin themselves seem to reduce magnesium status, possibly creating a vicious cycle.
In a 1993 trial, a low-magnesium diet reduced insulin sensitivity in healthy volunteers by 25% in just four weeks (8). It also increased urinary thromboxane concentration, a potential concern for cardiovascular health**.
At least three trials have shown that magnesium supplementation increases insulin sensitivity in insulin-resistant diabetics and non-diabetics (9, 10, 11). In some cases, the results were remarkable. In type II diabetics, 16 weeks of magnesium supplementation improved fasting glucose, calculated insulin sensitivity and HbA1c*** (12). HbA1c dropped by 22 percent.
In insulin resistant volunteers with low blood magnesium, magnesium supplementation for four months reduced estimated insulin resistance by 43 percent and decreased fasting insulin by 32 percent (13). This suggests to me that magnesium deficiency was probably one of the main reasons they were insulin resistant in the first place. But the study had another very interesting finding: magnesium improved the subjects' blood lipid profile remarkably. Total cholesterol decreased, LDL decreased, HDL increased and triglycerides decreased by a whopping 39 percent. The same thing had been reported in the medical literature decades earlier when doctors used magnesium injections to treat heart disease, and also in animals treated with magnesium. Magnesium supplementation also suppresses atherosclerosis (thickening and hardening of the arteries) in animal models, a fact that I may discuss in more detail at some point (14, 15).
In the previous study, participants were given 2.5 g magnesium chloride (MgCl2) per day. That's a bit more than the USDA recommended daily allowance (MgCl2 is mostly chloride by weight), in addition to what they were already getting from their diet. Most of a person's magnesium is in their bones, so correcting a deficiency by eating a nutritious diet may take a while.
Speaking of nutritious diets, how does one get magnesium? Good sources include halibut, leafy greens, chocolate and nuts. Bone broths may also be a source of magnesium. Whole grains and beans are also fairly good sources, while refined grains lack most of the magnesium in the whole grain. Organic foods, particularly artisanally produced foods from a farmer's market, are richer in magnesium because they grow on better soil and often use older varieties that are more nutritious.
The problem with seeds such as grains, beans and nuts is that they also contain phytic acid which prevents the absorption of magnesium and other minerals (16). Healthy non-industrial societies that relied on grains took great care in their preparation: they soaked them, often fermented them, and also frequently removed a portion of the bran before cooking (17). These steps all served to reduce the level of phytic acid and other anti-nutrients. I've posted a method for effectively reducing the amount of phytic acid in brown rice (18). Beans should ideally be soaked for 24 hours before cooking, preferably in warm water.
Industrial agriculture has systematically depleted our soil of many minerals, due to high-yield crop varieties and the fact that synthetic fertilizers only replace a few minerals. The mineral content of foods in the US, including magnesium, has dropped sharply in the last 50 years. The reason we need to use fertilizers in the first place is that we've broken the natural nutrient cycle in which minerals always return to the soil in the same place they were removed. In 21st century America, minerals are removed from the soil, pass through our toilets, and end up in the landfill or in waste water. This will continue until we find an acceptable way to return human feces and urine to agricultural soil, as many cultures do to this day****.
I believe that an adequate magnesium intake is critical for proper insulin sensitivity and overall health.
* Zucker rats that lack leptin signaling
** Thromboxane A2 is an omega-6 derived eicosanoid that potently constricts blood vessels and promotes blood clotting. It's interesting that magnesium has such a strong effect on it. It indicates that fatty acid balance is not the only major influence on eicosanoid production.
*** Glycated hemoglobin. A measure of the average blood glucose level over the past few weeks.
**** Anyone interested in further reading on this should look up The Humanure Handbook
During 1999–2000, the diet of a large proportion of the U.S. population did not contain adequate magnesium... Furthermore, racial or ethnic differences in magnesium persist and may contribute to some health disparities.... Because magnesium intake is low among many people in the United States and inadequate magnesium status is associated with increased risk of acute and chronic conditions, an urgent need exists to perform a current survey to assess the physiologic status of magnesium in the U.S. population.Magnesium is an essential mineral that many people apparently don't get enough of. One of the many things it's necessary for in mammals is proper insulin sensitivity and glucose control. A loss of glucose control due to insulin resistance can eventually lead to diabetes and all its complications.
Magnesium status is associated with insulin sensitivity (2, 3), and a low magnesium intake predicts the development of type II diabetes in most studies (4, 5) but not all (6). Magnesium supplements largely prevent diabetes in a rat model* (7). Interestingly, excess blood glucose and insulin themselves seem to reduce magnesium status, possibly creating a vicious cycle.
In a 1993 trial, a low-magnesium diet reduced insulin sensitivity in healthy volunteers by 25% in just four weeks (8). It also increased urinary thromboxane concentration, a potential concern for cardiovascular health**.
At least three trials have shown that magnesium supplementation increases insulin sensitivity in insulin-resistant diabetics and non-diabetics (9, 10, 11). In some cases, the results were remarkable. In type II diabetics, 16 weeks of magnesium supplementation improved fasting glucose, calculated insulin sensitivity and HbA1c*** (12). HbA1c dropped by 22 percent.
In insulin resistant volunteers with low blood magnesium, magnesium supplementation for four months reduced estimated insulin resistance by 43 percent and decreased fasting insulin by 32 percent (13). This suggests to me that magnesium deficiency was probably one of the main reasons they were insulin resistant in the first place. But the study had another very interesting finding: magnesium improved the subjects' blood lipid profile remarkably. Total cholesterol decreased, LDL decreased, HDL increased and triglycerides decreased by a whopping 39 percent. The same thing had been reported in the medical literature decades earlier when doctors used magnesium injections to treat heart disease, and also in animals treated with magnesium. Magnesium supplementation also suppresses atherosclerosis (thickening and hardening of the arteries) in animal models, a fact that I may discuss in more detail at some point (14, 15).
In the previous study, participants were given 2.5 g magnesium chloride (MgCl2) per day. That's a bit more than the USDA recommended daily allowance (MgCl2 is mostly chloride by weight), in addition to what they were already getting from their diet. Most of a person's magnesium is in their bones, so correcting a deficiency by eating a nutritious diet may take a while.
Speaking of nutritious diets, how does one get magnesium? Good sources include halibut, leafy greens, chocolate and nuts. Bone broths may also be a source of magnesium. Whole grains and beans are also fairly good sources, while refined grains lack most of the magnesium in the whole grain. Organic foods, particularly artisanally produced foods from a farmer's market, are richer in magnesium because they grow on better soil and often use older varieties that are more nutritious.
The problem with seeds such as grains, beans and nuts is that they also contain phytic acid which prevents the absorption of magnesium and other minerals (16). Healthy non-industrial societies that relied on grains took great care in their preparation: they soaked them, often fermented them, and also frequently removed a portion of the bran before cooking (17). These steps all served to reduce the level of phytic acid and other anti-nutrients. I've posted a method for effectively reducing the amount of phytic acid in brown rice (18). Beans should ideally be soaked for 24 hours before cooking, preferably in warm water.
Industrial agriculture has systematically depleted our soil of many minerals, due to high-yield crop varieties and the fact that synthetic fertilizers only replace a few minerals. The mineral content of foods in the US, including magnesium, has dropped sharply in the last 50 years. The reason we need to use fertilizers in the first place is that we've broken the natural nutrient cycle in which minerals always return to the soil in the same place they were removed. In 21st century America, minerals are removed from the soil, pass through our toilets, and end up in the landfill or in waste water. This will continue until we find an acceptable way to return human feces and urine to agricultural soil, as many cultures do to this day****.
I believe that an adequate magnesium intake is critical for proper insulin sensitivity and overall health.
* Zucker rats that lack leptin signaling
** Thromboxane A2 is an omega-6 derived eicosanoid that potently constricts blood vessels and promotes blood clotting. It's interesting that magnesium has such a strong effect on it. It indicates that fatty acid balance is not the only major influence on eicosanoid production.
*** Glycated hemoglobin. A measure of the average blood glucose level over the past few weeks.
**** Anyone interested in further reading on this should look up The Humanure Handbook
Lindeberg on Obesity
I'm currently reading Dr. Staffan Lindeberg's magnum opus Food and Western Disease, recently published in English for the first time. Dr. Lindeberg is one of the world's leading experts on the health and diet of non-industrial cultures, particularly in Papua New Guinea. The book contains 2,034 references. It's also full of quotable statements. Here's what he has to say about obesity:
I'd recommend this book to anyone who has a scholarly interest in health and nutrition, and somewhat of a background in science and medicine. It's extremely well referenced, which makes it much more valuable.
Middle-age spread is a normal phenomenon - assuming you live in the West. Few people are able to maintain their [youthful] waistline after age 50. The usual explanation - too little exercise and too much food - does not fully take into account the situation among traditional populations. Such people are usually not as physically active as you may think, and they usually eat large quantities of food.The only obese Kitavans Dr. Lindeberg observed were two people who had spent several years off the island living a modern, urban lifestyle, and were back on Kitava for a visit.
Overweight has been extremely rare among hunter-gatherers and other traditional cultures [18 references]. This simple fact has been quickly apparent to all foreign visitors...
The Kitava study measured height, weight, waist circumference, subcutaneous fat thickness at the back of the upper arm (triceps skinfold) and upper arm circumference on 272 persons ages 4-86 years. Overweight and obesity were absent and average [body mass index] was low across all age groups. ...no one was larger around their waist than around their hips.
...The circumference of the upper arm [mostly indicating muscle mass] was only negligibly smaller on Kitava [compared with Sweden], which indicates that there was no malnutrition. It is obvious from our investigations that lack of food is an unknown concept, and that the surplus of fruits and vegetables regularly rots or is eaten by dogs.
The Population of Kitava occupies a unique position in the world in terms of the negligible effect that the Western lifestyle has had on the island.
I'd recommend this book to anyone who has a scholarly interest in health and nutrition, and somewhat of a background in science and medicine. It's extremely well referenced, which makes it much more valuable.
Saturday, February 20, 2010
What should be my HDL cholesterol?
HDL cholesterol levels are a rough measure of HDL particle quantity in the blood. They actually tell us next to nothing about HDL particle type, although HDL cholesterol increases are usually associated with increases in LDL particle size. This a good thing, since small-dense LDL particles are associated with increased cardiovascular disease.
Most blood lipid panels reviewed by family doctors with patients give information about HDL status through measures of HDL cholesterol, provided in one of the standard units (e.g., mg/dl).
Study after study shows that HDL cholesterol levels, although imprecise, are a much better predictor of cardiovascular disease than LDL or total cholesterol levels. How high should be one’s HDL cholesterol? The answer to this question is somewhat dependent on each individual’s health profile, but most data suggest that a level greater than 60 mg/dl (1.55 mmol/l) is close to optimal for most people.
The figure below (from Eckardstein, 2008; full reference at the end of this post) plots incidence of coronary events in men (on the vertical axis), over a period of 10 years, against HDL cholesterol levels (on the horizontal axis). Note: IFG = impaired fasting glucose. This relationship is similar for women, particularly post-menopausal women. Pre-menopausal women usually have higher HDL cholesterol levels than men, and a low incidence of coronary events.
From the figure above, one can say that a diabetic man with about 55 mg/dl of HDL cholesterol will have approximately the same chance, on average, of having a coronary event (a heart attack) as a man with no risk factors and about 20 mg/dl of HDL cholesterol. That chance will be about 7 percent. With 20 mg/dl of HDL cholesterol, the chance of a diabetic man having a coronary event would approach 50 percent.
We can also conclude from the figure above that a man with no risk factors will have a 5 percent chance of having a coronary event if his HDL cholesterol is about 25 mg/dl; and about 2 percent if his HDL cholesterol is greater than 60 mg/dl. This a 60 percent reduction in risk, a risk that was low to start with because of the absence of risk factors.
HDL cholesterol levels greater than 60 are associated with significantly reduced risks of coronary events, particularly for those with diabetes (the graph does not take diabetes type into consideration). Much higher levels of HDL cholesterol (beyond 60) do not seem to be associated with much lower risk of coronary events.
Conversely, a very low HDL cholesterol level (below 25) is a major risk factor when other risk factors are also present, particularly: diabetes, hypertension (high blood pressure), and familial hypercholesteromia (gene-induced very elevated LDL cholesterol).
It is not yet clear whether HDL cholesterol is a cause of reduced cardiovascular disease, or just a marker of other health factors that lead to reduced risk for cardiovascular disease. Much of the empirical evidence suggests a causal relationship, and if this is the case then it may be a good idea to try to increase HDL levels. Even if HDL cholesterol is just a marker, the same strategy that increases it may also have a positive impact on the real causative factor of which HDL cholesterol is a marker.
What can one do to increase his or her HDL cholesterol? One way is to replace refined carbs and sugars with saturated fat and cholesterol in one’s diet. (I know that this sounds counterintuitive, but seems to work.) Another is to increase one’s vitamin D status, through sun exposure or supplementation.
Other therapeutic interventions can also be used to increase HDL; some more natural than others. The figure below (also from Eckardstein, 2008) shows the maximum effects of several therapeutic interventions to increase HDL cholesterol.
Among the therapeutic interventions shown in the figure above, taking nicotinic acid (niacin) in pharmacological doses, of 1 to 3 g per day (higher dosages may be toxic), is by far the most effective way of increasing one’s HDL cholesterol. Only the niacin that causes flush is effective in this respect. No-flush niacin preparations may have some anti-inflammatory effects, but do not cause increases in HDL cholesterol.
Rimonabant, which is second to niacin in its effect on HDL cholesterol, is an appetite suppressor that has been associated with serious side effects and, to be best of my knowledge, has been largely banned from use in pharmaceutical drugs.
Third in terms of effectiveness, among the factors shown in the figure, is moderate alcohol consumption. Running about 19 miles per week (2.7 miles per day) and taking fibrates are tied in forth place.
Many people think that they are having a major allergic reaction, and have a panic attack, when they experience the niacin flush. This usually happens several minutes after taking niacin, and depends on the dose and whether niacin was consumed with food or not. It is not uncommon for one’s entire torso to turn hot red, as though the person had had major sunburn. This reaction is harmless, and usually disappears after several minutes.
One could say that, with niacin: no “pain” (i.e., flush), no gain.
Reference:
von Eckardstein, A. (2008). HDL – a difficult friend. Drug Discovery Today: Disease Mechanisms, 5(3), 315-324.
Most blood lipid panels reviewed by family doctors with patients give information about HDL status through measures of HDL cholesterol, provided in one of the standard units (e.g., mg/dl).
Study after study shows that HDL cholesterol levels, although imprecise, are a much better predictor of cardiovascular disease than LDL or total cholesterol levels. How high should be one’s HDL cholesterol? The answer to this question is somewhat dependent on each individual’s health profile, but most data suggest that a level greater than 60 mg/dl (1.55 mmol/l) is close to optimal for most people.
The figure below (from Eckardstein, 2008; full reference at the end of this post) plots incidence of coronary events in men (on the vertical axis), over a period of 10 years, against HDL cholesterol levels (on the horizontal axis). Note: IFG = impaired fasting glucose. This relationship is similar for women, particularly post-menopausal women. Pre-menopausal women usually have higher HDL cholesterol levels than men, and a low incidence of coronary events.
From the figure above, one can say that a diabetic man with about 55 mg/dl of HDL cholesterol will have approximately the same chance, on average, of having a coronary event (a heart attack) as a man with no risk factors and about 20 mg/dl of HDL cholesterol. That chance will be about 7 percent. With 20 mg/dl of HDL cholesterol, the chance of a diabetic man having a coronary event would approach 50 percent.
We can also conclude from the figure above that a man with no risk factors will have a 5 percent chance of having a coronary event if his HDL cholesterol is about 25 mg/dl; and about 2 percent if his HDL cholesterol is greater than 60 mg/dl. This a 60 percent reduction in risk, a risk that was low to start with because of the absence of risk factors.
HDL cholesterol levels greater than 60 are associated with significantly reduced risks of coronary events, particularly for those with diabetes (the graph does not take diabetes type into consideration). Much higher levels of HDL cholesterol (beyond 60) do not seem to be associated with much lower risk of coronary events.
Conversely, a very low HDL cholesterol level (below 25) is a major risk factor when other risk factors are also present, particularly: diabetes, hypertension (high blood pressure), and familial hypercholesteromia (gene-induced very elevated LDL cholesterol).
It is not yet clear whether HDL cholesterol is a cause of reduced cardiovascular disease, or just a marker of other health factors that lead to reduced risk for cardiovascular disease. Much of the empirical evidence suggests a causal relationship, and if this is the case then it may be a good idea to try to increase HDL levels. Even if HDL cholesterol is just a marker, the same strategy that increases it may also have a positive impact on the real causative factor of which HDL cholesterol is a marker.
What can one do to increase his or her HDL cholesterol? One way is to replace refined carbs and sugars with saturated fat and cholesterol in one’s diet. (I know that this sounds counterintuitive, but seems to work.) Another is to increase one’s vitamin D status, through sun exposure or supplementation.
Other therapeutic interventions can also be used to increase HDL; some more natural than others. The figure below (also from Eckardstein, 2008) shows the maximum effects of several therapeutic interventions to increase HDL cholesterol.
Among the therapeutic interventions shown in the figure above, taking nicotinic acid (niacin) in pharmacological doses, of 1 to 3 g per day (higher dosages may be toxic), is by far the most effective way of increasing one’s HDL cholesterol. Only the niacin that causes flush is effective in this respect. No-flush niacin preparations may have some anti-inflammatory effects, but do not cause increases in HDL cholesterol.
Rimonabant, which is second to niacin in its effect on HDL cholesterol, is an appetite suppressor that has been associated with serious side effects and, to be best of my knowledge, has been largely banned from use in pharmaceutical drugs.
Third in terms of effectiveness, among the factors shown in the figure, is moderate alcohol consumption. Running about 19 miles per week (2.7 miles per day) and taking fibrates are tied in forth place.
Many people think that they are having a major allergic reaction, and have a panic attack, when they experience the niacin flush. This usually happens several minutes after taking niacin, and depends on the dose and whether niacin was consumed with food or not. It is not uncommon for one’s entire torso to turn hot red, as though the person had had major sunburn. This reaction is harmless, and usually disappears after several minutes.
One could say that, with niacin: no “pain” (i.e., flush), no gain.
Reference:
von Eckardstein, A. (2008). HDL – a difficult friend. Drug Discovery Today: Disease Mechanisms, 5(3), 315-324.
Labels:
cholesterol,
HDL,
LDL,
niacin,
research,
saturated fat
Tuesday, February 16, 2010
Large LDL and small HDL particles: The best combination
High-density lipoprotein (HDL) is one of the five main types of lipoproteins found in circulation, together with very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL), and chylomicrons.
After a fatty meal, the blood is filled with chylomicrons, which carry triglycerides (TGAs). The TGAs are transferred to cells from chylomicrons via the activity of enzymes, in the form of free fatty acids (FFAs), which are used by those cells as sources of energy.
After delivering FFAs to the cells, the chylomicrons progressively lose their TGA content and “shrink”, eventually being absorbed and recycled by the liver. The liver exports part of the TGAs that it gets from chylomicrons back to cells for use as energy as well, now in the form of VLDL. As VLDL particles deliver TGAs to the cells they shrink in size, similarly to chylomicrons. As they shrink, VLDL particles first become IDL and then LDL particles.
The figure below (click on it to enlarge), from Elliott & Elliott (2009; reference at the end of this post), shows, on the same scale: (a) VLDL particles, (b) chylomicrons, (c) LDL particles, and (d) HDL particles. The dark bar at the bottom of each shot is 1000 A in length, or 100 nm (A = angstrom; nm = nanometer; 1 nm = 10 A).
As you can see from the figure, most of the LDL particles shown are about 1/4 of the length of the dark bar in diameter, often slightly more, or about 25-27 nm in size. They come in different sizes, with sizes in this range being the most common. The smaller and denser they are, the more likely they are to contribute to the formation of atherosclerotic plaque in the presence of other factors, such as chronic inflammation. The larger they become, which usually happens in diets high in saturated fat, the less likely they are to form plaque.
Note that the HDL particles are rather small compared to the LDL particles. Shouldn’t they cause plaque then? Not really. Apparently they have to be small, compared to LDL particles, to do their job effectively.
HDL is a completely different animal from VLDL, IDL and LDL. HDL particles are produced by the liver as dense disk-like particles, known as nascent HDL particles. These nascent HDL particles progressively pick up cholesterol from cells, as well as performing a number of other functions, and “fatten up” with cholesterol in the process.
This process also involves HDL particles picking up cholesterol from plaque in the artery walls, which is one of the reasons why HDL cholesterol is informally called “good” cholesterol. In fact, neither HDL nor LDL are really cholesterol; HDL and LDL are particles that carry cholesterol, protein and fat.
As far as particle size is concerned, LDL and HDL are opposites. Large LDL particles are the least likely to cause plaque formation, because LDL particles have to be approximately 25 nm in diameter or smaller to penetrate the artery walls. With HDL the opposite seems to be true, as HDL particles need to be small (compared with LDL particles) to easily penetrate the artery walls in order to pick up cholesterol, leave the artery walls with their cargo, and have it returned back to the liver.
Interestingly, some research suggests HDL particles that are larger in size, when compared with other HDL particles (not with LDL particles), seem to do a better job than very small HDL particles in terms of reducing risk of cardiovascular disease. It is also possible that a high number of larger HDL particles in the blood is indicative of elevated levels of "effective" HDL particles; i.e., particles that are effective at picking up cholesterol from the artery walls in the first place.
Another interesting aspect of this cycle is that the return to the liver of cholesterol picked up by HDL appears to be done largely via IDL and LDL particles (Elliott & Elliott, 2009), which get the cholesterol directly from HDL particles! Life is not that simple.
Reference:
William H. Elliott & Daphne C. Elliott (2009). Biochemistry and Molecular Biology. 4th Edition. New York: NY: Oxford University Press.
After a fatty meal, the blood is filled with chylomicrons, which carry triglycerides (TGAs). The TGAs are transferred to cells from chylomicrons via the activity of enzymes, in the form of free fatty acids (FFAs), which are used by those cells as sources of energy.
After delivering FFAs to the cells, the chylomicrons progressively lose their TGA content and “shrink”, eventually being absorbed and recycled by the liver. The liver exports part of the TGAs that it gets from chylomicrons back to cells for use as energy as well, now in the form of VLDL. As VLDL particles deliver TGAs to the cells they shrink in size, similarly to chylomicrons. As they shrink, VLDL particles first become IDL and then LDL particles.
The figure below (click on it to enlarge), from Elliott & Elliott (2009; reference at the end of this post), shows, on the same scale: (a) VLDL particles, (b) chylomicrons, (c) LDL particles, and (d) HDL particles. The dark bar at the bottom of each shot is 1000 A in length, or 100 nm (A = angstrom; nm = nanometer; 1 nm = 10 A).
As you can see from the figure, most of the LDL particles shown are about 1/4 of the length of the dark bar in diameter, often slightly more, or about 25-27 nm in size. They come in different sizes, with sizes in this range being the most common. The smaller and denser they are, the more likely they are to contribute to the formation of atherosclerotic plaque in the presence of other factors, such as chronic inflammation. The larger they become, which usually happens in diets high in saturated fat, the less likely they are to form plaque.
Note that the HDL particles are rather small compared to the LDL particles. Shouldn’t they cause plaque then? Not really. Apparently they have to be small, compared to LDL particles, to do their job effectively.
HDL is a completely different animal from VLDL, IDL and LDL. HDL particles are produced by the liver as dense disk-like particles, known as nascent HDL particles. These nascent HDL particles progressively pick up cholesterol from cells, as well as performing a number of other functions, and “fatten up” with cholesterol in the process.
This process also involves HDL particles picking up cholesterol from plaque in the artery walls, which is one of the reasons why HDL cholesterol is informally called “good” cholesterol. In fact, neither HDL nor LDL are really cholesterol; HDL and LDL are particles that carry cholesterol, protein and fat.
As far as particle size is concerned, LDL and HDL are opposites. Large LDL particles are the least likely to cause plaque formation, because LDL particles have to be approximately 25 nm in diameter or smaller to penetrate the artery walls. With HDL the opposite seems to be true, as HDL particles need to be small (compared with LDL particles) to easily penetrate the artery walls in order to pick up cholesterol, leave the artery walls with their cargo, and have it returned back to the liver.
Interestingly, some research suggests HDL particles that are larger in size, when compared with other HDL particles (not with LDL particles), seem to do a better job than very small HDL particles in terms of reducing risk of cardiovascular disease. It is also possible that a high number of larger HDL particles in the blood is indicative of elevated levels of "effective" HDL particles; i.e., particles that are effective at picking up cholesterol from the artery walls in the first place.
Another interesting aspect of this cycle is that the return to the liver of cholesterol picked up by HDL appears to be done largely via IDL and LDL particles (Elliott & Elliott, 2009), which get the cholesterol directly from HDL particles! Life is not that simple.
Reference:
William H. Elliott & Daphne C. Elliott (2009). Biochemistry and Molecular Biology. 4th Edition. New York: NY: Oxford University Press.
Saturday, February 13, 2010
Want to improve your cholesterol profile? Replace refined carbs and sugars with saturated fat and cholesterol in your diet
An interesting study by Clifton and colleagues (1998; full reference and link at the end of this post) looked at whether LDL cholesterol particle size distribution at baseline (i.e., beginning of the study) for various people was a determinant of lipid profile changes in each of two diets – one low and the other high in fat. This study highlights a few interesting points made in a previous post, which are largely unrelated to the main goal or findings of the study, but that are supported by side findings:
- As one increases dietary cholesterol and fat consumption, particularly saturated fat, circulating HDL cholesterol increases significantly. This happens whether one is taking niacin or not, although niacin seems to help, possibly as an independent (not moderating) factor. Increasing serum vitamin D levels, which can be done through sunlight exposure and supplementation, are also known to increase circulating HDL cholesterol.
- As one increases dietary cholesterol and fat consumption, particularly saturated fat, triglycerides in the fasting state (i.e., measured after a 8-hour fast) decrease significantly, particularly on a low carbohydrate diet. Triglycerides in the fasting state are negatively correlated with HDL cholesterol; they go down as HDL cholesterol goes up. This happens whether one is taking niacin or supplementing omega 3 fats or not, although these seem to help, possibly as independent factors.
- If one increases dietary fat intake, without also decreasing carbohydrate intake (particularly in the form of refined grains and sugars), LDL cholesterol will increase. Even so, LDL particle sizes will shift to more benign forms, which are the larger forms. Not all LDL particles change to benign forms, and there seem to be some genetic factors that influence this. LDL particles larger than 26 nm in diameter simply cannot pass through the gaps in the endothelium, which is a thin layer of cells lining the interior surface of arteries, and thus do not induce plaque formation.
The study by Clifton and colleagues (1998) involved 54 men and 51 women with a wide range of lipid profiles. They first underwent a 2-week low fat period, after which they were given two liquid supplements in addition to their low fat diet, for a period of 3 weeks. One of the liquid supplements contained 31 to 40 g of fat, and 650 to 845 mg of cholesterol. The other was fat and cholesterol free.
Studies that adopt a particular diet at baseline have the advantage of departing from a uniform diet across conditions. They also typically have one common characteristic: the baseline diet reflects the beliefs of the authors about what an ideal diet is. That is not always the case, of course. If this was indeed the case here, we have a particularly interesting study, because in that case the side findings discussed below contradicted the authors’ beliefs.
The table below shows the following measures for the participants in the study: age, body mass index (BMI), waist-to-hip ratio (WHR), total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, and three subtypes of high-density lipoprotein (HDL) cholesterol. LDL cholesterol is the colloquially known as the “bad” type, and “HDL” as the good one (which is an oversimplification). In short, the participants were overweight, middle-aged men and women, with relatively poor lipid profiles.
At the bottom of the table is the note “P < 0.001”, following a small “a”. This essentially means that on the rows indicated by an “a”, like the “WHR” row, the difference in the averages (e.g., 0.81 for women, and 0.93 for men, in the WHR row) was significantly different from what one would expect it to be due to chance alone. More precisely, the likelihood that the difference was due to chance was lower than 0.001, or 0.1 percent, in the case of a P < 0.001. Usually a difference between averages (a.k.a. means) associated with a P < 0.05 will be considered statistically significant.
Since the LDL cholesterol concentrations (as well as other lipoprotein concentrations) are listed on the table in mmol/L, and many people receive those measures in mg/dL in blood lipid profile test reports, below is a conversion table for LDL cholesterol (from: Wikipedia).
The table below shows the dietary intake in the low and high fat diets. Note that in the high fat diet, not only is the fat intake higher, but so is the cholesterol intake. The latter is significantly higher, more than 4 times the intake in the low fat diet, and about 2.5 times the recommended daily value by the U.S. Food and Drug Administration. The total calorie intake is reported as slightly lower in the high fat diet than in the low fat diet.
Note that the largest increase was in saturated fat, followed by an almost equally large increase in monounsaturated fat. This, together with the increase in cholesterol, mimics a move to a diet where fatty meat and organs are consumed in higher quantities, with a corresponding reduction in the intake of refined carbohydrates (e.g., bread, pasta, sugar, potatoes) and lean meats.
Finally, the table below shows the changes in lipid profiles in the low and high fat diets. Note that all subtypes of HDL (or "good") cholesterol concentrations were significantly higher in the high fat diet, which is very telling, because HDL cholesterol concentrations are much better predictors of cardiovascular disease than LDL or total cholesterol concentrations. The higher the HDL cholesterol, the lower the risk of cardiovascular disease.
In the table above, we also see that triglycerides are significantly lower in the high fat diet, which is also good, because high fasting triglyceride concentrations are associated with cardiovascular disease and also insulin resistance (which is associated with diabetes).
However, the total and LDL cholesterol were also significantly higher in the high fat compared to the low fat diet. Is this as bad as it sounds? Not when we look at other factors that are not clear from the tables in the article.
One of those factors is the likely change in LDL particle size. LDL particle sizes almost always increase with significant increases in HDL; frequently going up in diameter beyond 26 nm, and thus passing the threshold beyond which an LDL particle can penetrate the endothelium and help form a plaque.
Another important factor to take into consideration is the somewhat strange decision by the authors to use the Friedewald equation to estimate the LDL concentrations in the low and high fat diets. Through the Friedewald equation, LDL is calculated as follows (where TC is total cholesterol):
LDL = TC – HDL – Triglycerides / 5
Here is one of the problems with the Friedewald equation. Let us assume that an individual has the following lipid profile numbers: TC = 200, HDL = 50, and trigs. = 150. The calculated LDL will be 120. Let us assume that this same individual reduces trigs. to 50, from the previous 150, keeping all of the other measures constant. This is a major improvement. Yet, the calculated LDL will now be 140, and a doctor will tell this person to consider taking statins!
By the way, most people who do a blood test and get their lipid profile report also get their LDL calculated through the Friedewald equation. Usually this is indicated through a "CALC" note next to the description of the test or the calculated LDL number.
Finally, total cholesterol is not a very useful measure, because an elevated total cholesterol may be primarily reflecting an elevated HDL, which is healthy. Also, a slightly elevated total cholesterol seems to be protective, as it is associated with reduced overall mortality and also reduced mortality from cardiovascular disease, according to U-curve regression studies comparing mortality and total cholesterol levels in different countries.
We do not know for sure that the participants in this study were consuming a lot of refined carbohydrates and/or sugars at baseline. But it is a safe bet that they were, since they were consuming 214 g of carbohydrates per day. It is difficult, although not impossible, to eat that many carbohydrates per day by eating only vegetables and fruits, which are mostly water. Consumption of starches makes it easier to reach that level.
This is why when one goes on a paleo diet, he or she reduces significantly the amount of dietary carbohydrates; even more so on a targeted low carbohydrate diet, such as the Atkins diet. Richard K. Bernstein, who is a type 1 diabetic and has been adopting a strict low carbohydrate diet during most of his adult life, had the following lipid profile at 72 years of age: HDL = 118, LDL = 53, trigs. = 45. His fasting blood sugar was reportedly 83 mg/dl. Click here to listen to an interview with Dr. Bernstein on the The Livin' La Vida Low-Carb Show.
The lipid profile improvement observed (e.g., a 14 percent increase in HDL from baseline for men, and about half that for women, in only 3 weeks) was very likely due to an increase in dietary saturated fat and cholesterol combined with a decrease in refined carbohydrates and sugars. The improvement would probably have been even more impressive with a higher increase in saturated fat, as long as it was accompanied by the elimination of refined carbohydrates and sugars from the participants’ diets.
Reference:
Clifton, P. M., M. Noakes, and P. J. Nestel (1998). LDL particle size and LDL and HDL cholesterol changes with dietary fat and cholesterol in healthy subjects. J. Lipid. Res. 39: 1799–1804.
- As one increases dietary cholesterol and fat consumption, particularly saturated fat, circulating HDL cholesterol increases significantly. This happens whether one is taking niacin or not, although niacin seems to help, possibly as an independent (not moderating) factor. Increasing serum vitamin D levels, which can be done through sunlight exposure and supplementation, are also known to increase circulating HDL cholesterol.
- As one increases dietary cholesterol and fat consumption, particularly saturated fat, triglycerides in the fasting state (i.e., measured after a 8-hour fast) decrease significantly, particularly on a low carbohydrate diet. Triglycerides in the fasting state are negatively correlated with HDL cholesterol; they go down as HDL cholesterol goes up. This happens whether one is taking niacin or supplementing omega 3 fats or not, although these seem to help, possibly as independent factors.
- If one increases dietary fat intake, without also decreasing carbohydrate intake (particularly in the form of refined grains and sugars), LDL cholesterol will increase. Even so, LDL particle sizes will shift to more benign forms, which are the larger forms. Not all LDL particles change to benign forms, and there seem to be some genetic factors that influence this. LDL particles larger than 26 nm in diameter simply cannot pass through the gaps in the endothelium, which is a thin layer of cells lining the interior surface of arteries, and thus do not induce plaque formation.
The study by Clifton and colleagues (1998) involved 54 men and 51 women with a wide range of lipid profiles. They first underwent a 2-week low fat period, after which they were given two liquid supplements in addition to their low fat diet, for a period of 3 weeks. One of the liquid supplements contained 31 to 40 g of fat, and 650 to 845 mg of cholesterol. The other was fat and cholesterol free.
Studies that adopt a particular diet at baseline have the advantage of departing from a uniform diet across conditions. They also typically have one common characteristic: the baseline diet reflects the beliefs of the authors about what an ideal diet is. That is not always the case, of course. If this was indeed the case here, we have a particularly interesting study, because in that case the side findings discussed below contradicted the authors’ beliefs.
The table below shows the following measures for the participants in the study: age, body mass index (BMI), waist-to-hip ratio (WHR), total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, and three subtypes of high-density lipoprotein (HDL) cholesterol. LDL cholesterol is the colloquially known as the “bad” type, and “HDL” as the good one (which is an oversimplification). In short, the participants were overweight, middle-aged men and women, with relatively poor lipid profiles.
At the bottom of the table is the note “P < 0.001”, following a small “a”. This essentially means that on the rows indicated by an “a”, like the “WHR” row, the difference in the averages (e.g., 0.81 for women, and 0.93 for men, in the WHR row) was significantly different from what one would expect it to be due to chance alone. More precisely, the likelihood that the difference was due to chance was lower than 0.001, or 0.1 percent, in the case of a P < 0.001. Usually a difference between averages (a.k.a. means) associated with a P < 0.05 will be considered statistically significant.
Since the LDL cholesterol concentrations (as well as other lipoprotein concentrations) are listed on the table in mmol/L, and many people receive those measures in mg/dL in blood lipid profile test reports, below is a conversion table for LDL cholesterol (from: Wikipedia).
The table below shows the dietary intake in the low and high fat diets. Note that in the high fat diet, not only is the fat intake higher, but so is the cholesterol intake. The latter is significantly higher, more than 4 times the intake in the low fat diet, and about 2.5 times the recommended daily value by the U.S. Food and Drug Administration. The total calorie intake is reported as slightly lower in the high fat diet than in the low fat diet.
Note that the largest increase was in saturated fat, followed by an almost equally large increase in monounsaturated fat. This, together with the increase in cholesterol, mimics a move to a diet where fatty meat and organs are consumed in higher quantities, with a corresponding reduction in the intake of refined carbohydrates (e.g., bread, pasta, sugar, potatoes) and lean meats.
Finally, the table below shows the changes in lipid profiles in the low and high fat diets. Note that all subtypes of HDL (or "good") cholesterol concentrations were significantly higher in the high fat diet, which is very telling, because HDL cholesterol concentrations are much better predictors of cardiovascular disease than LDL or total cholesterol concentrations. The higher the HDL cholesterol, the lower the risk of cardiovascular disease.
In the table above, we also see that triglycerides are significantly lower in the high fat diet, which is also good, because high fasting triglyceride concentrations are associated with cardiovascular disease and also insulin resistance (which is associated with diabetes).
However, the total and LDL cholesterol were also significantly higher in the high fat compared to the low fat diet. Is this as bad as it sounds? Not when we look at other factors that are not clear from the tables in the article.
One of those factors is the likely change in LDL particle size. LDL particle sizes almost always increase with significant increases in HDL; frequently going up in diameter beyond 26 nm, and thus passing the threshold beyond which an LDL particle can penetrate the endothelium and help form a plaque.
Another important factor to take into consideration is the somewhat strange decision by the authors to use the Friedewald equation to estimate the LDL concentrations in the low and high fat diets. Through the Friedewald equation, LDL is calculated as follows (where TC is total cholesterol):
LDL = TC – HDL – Triglycerides / 5
Here is one of the problems with the Friedewald equation. Let us assume that an individual has the following lipid profile numbers: TC = 200, HDL = 50, and trigs. = 150. The calculated LDL will be 120. Let us assume that this same individual reduces trigs. to 50, from the previous 150, keeping all of the other measures constant. This is a major improvement. Yet, the calculated LDL will now be 140, and a doctor will tell this person to consider taking statins!
By the way, most people who do a blood test and get their lipid profile report also get their LDL calculated through the Friedewald equation. Usually this is indicated through a "CALC" note next to the description of the test or the calculated LDL number.
Finally, total cholesterol is not a very useful measure, because an elevated total cholesterol may be primarily reflecting an elevated HDL, which is healthy. Also, a slightly elevated total cholesterol seems to be protective, as it is associated with reduced overall mortality and also reduced mortality from cardiovascular disease, according to U-curve regression studies comparing mortality and total cholesterol levels in different countries.
We do not know for sure that the participants in this study were consuming a lot of refined carbohydrates and/or sugars at baseline. But it is a safe bet that they were, since they were consuming 214 g of carbohydrates per day. It is difficult, although not impossible, to eat that many carbohydrates per day by eating only vegetables and fruits, which are mostly water. Consumption of starches makes it easier to reach that level.
This is why when one goes on a paleo diet, he or she reduces significantly the amount of dietary carbohydrates; even more so on a targeted low carbohydrate diet, such as the Atkins diet. Richard K. Bernstein, who is a type 1 diabetic and has been adopting a strict low carbohydrate diet during most of his adult life, had the following lipid profile at 72 years of age: HDL = 118, LDL = 53, trigs. = 45. His fasting blood sugar was reportedly 83 mg/dl. Click here to listen to an interview with Dr. Bernstein on the The Livin' La Vida Low-Carb Show.
The lipid profile improvement observed (e.g., a 14 percent increase in HDL from baseline for men, and about half that for women, in only 3 weeks) was very likely due to an increase in dietary saturated fat and cholesterol combined with a decrease in refined carbohydrates and sugars. The improvement would probably have been even more impressive with a higher increase in saturated fat, as long as it was accompanied by the elimination of refined carbohydrates and sugars from the participants’ diets.
Reference:
Clifton, P. M., M. Noakes, and P. J. Nestel (1998). LDL particle size and LDL and HDL cholesterol changes with dietary fat and cholesterol in healthy subjects. J. Lipid. Res. 39: 1799–1804.
Tuesday, February 9, 2010
Saturated Fat and Insulin Sensitivity
Insulin sensitivity is a measure of the tissue response to insulin. Typically, it refers to insulin's ability to cause tissues to absorb glucose from the blood. A loss of insulin sensitivity, also called insulin resistance, is a core part of the metabolic disorder that affects many people in industrial nations.
It is commonly asserted in journal articles and on the internet that saturated fat reduces insulin sensitivity. The idea is that saturated fat reduces the body's ability to handle glucose effectively, placing people on the road to diabetes, obesity and heart disease. Perhaps this particular claim deserves a closer look.
The Evidence
I found a review article from 2008 that addressed this question (1). I like this review because it only includes high-quality trials that used reliable methods of determining insulin sensitivity*.
On to the meat of it. There were 5 studies in which non-diabetic people were fed diets rich in saturated fat, and compared with a group eating a diet rich in monounsaturated (like olive oil) or polyunsaturated (like corn oil) fat. They ranged in duration from one week to 3 months. Four of the five studies found that fat quality did not affect insulin sensitivity, including one of the 3-month studies.
The fifth study, which is the one that's most commonly cited, requires some discussion. This was the KANWU study (2). Over the course of three months, investigators fed 163 volunteers a diet rich in either saturated fat or monounsaturated fat.
What the authors focused on is the fact that insulin sensitivity declined slightly but significantly on the saturated fat diet compared with the pre-diet baseline. That's why this study is cited as evidence that saturated fat impairs insulin sensitivity. But those of you with a science background may be able to spot the problem here. You need a control group for comparison, to take into account normal fluctuations caused by such things as the season, eating a new diet provided by the investigators, and having a doctor poking at you. That control group was the group eating monounsaturated fat. The comparison between diet groups was the comparison that matters most, and it wasn't quite significant. I think the most you can say about this study is that it provides weak evidence that saturated fat decreases insulin sensitivity.
So we have five studies through 2008, which overall offer little support the idea that saturated fat reduces insulin sensitivity in non-diabetics. Since the review paper was published, I know of one subsequent study that asked the same question (3). Susan J. van Dijk and colleagues fed volunteers with abdominal overweight a diet rich in either saturated fat or monounsaturated fat. I e-mailed the senior author and she said the saturated fat diet was "mostly butter". After 8 weeks, insulin sensitivity was virtually identical between the two groups. This study appeared well controlled and used the gold standard method for assessing insulin sensitivity, called the euglycemic-hyperinsulinemic clamp technique***.
The evidence from controlled trials is rather consistent that saturated fat has no major effect on insulin sensitivity in humans, at least on time scales of a few months.
UPDATE: other trials have added to this finding. The large European LIPIGENE randomized controlled diet trial found that substantial differences in SFA intake had no effect on insulin sensitivity over 12 weeks in people with the metabolic syndrome (3b).
* For the nerds: euglycemic-hyperinsulinemic clamp (the gold standard), insulin suppression test, or intravenous glucose tolerance test with Minimal Model. They didn't include studies that reported HOMA as their only measure, because it's not very accurate.
*** They did find that markers of inflammation in fat tissue were higher after the saturated fat diet.
It is commonly asserted in journal articles and on the internet that saturated fat reduces insulin sensitivity. The idea is that saturated fat reduces the body's ability to handle glucose effectively, placing people on the road to diabetes, obesity and heart disease. Perhaps this particular claim deserves a closer look.
The Evidence
I found a review article from 2008 that addressed this question (1). I like this review because it only includes high-quality trials that used reliable methods of determining insulin sensitivity*.
On to the meat of it. There were 5 studies in which non-diabetic people were fed diets rich in saturated fat, and compared with a group eating a diet rich in monounsaturated (like olive oil) or polyunsaturated (like corn oil) fat. They ranged in duration from one week to 3 months. Four of the five studies found that fat quality did not affect insulin sensitivity, including one of the 3-month studies.
The fifth study, which is the one that's most commonly cited, requires some discussion. This was the KANWU study (2). Over the course of three months, investigators fed 163 volunteers a diet rich in either saturated fat or monounsaturated fat.
The SAFA diet included butter and a table margarine containing a relatively high proportion of SAFAs. The MUFA diet included a spread and a margarine containing high proportions of oleic acid derived from high-oleic sunflower oil and negligible amounts of trans fatty acids and n-3 fatty acids and olive oil.Yummy. After three months of these diets, there was no significant difference in insulin sensitivity between the saturated fat group and the monounsaturated fat group. Yes, you read that right. Even the study that's commonly cited as evidence that saturated fat causes insulin resistance found no significant difference between the diets. I'll be generous and acknowledge that the small difference was almost statistically significant (p = 0.053).
What the authors focused on is the fact that insulin sensitivity declined slightly but significantly on the saturated fat diet compared with the pre-diet baseline. That's why this study is cited as evidence that saturated fat impairs insulin sensitivity. But those of you with a science background may be able to spot the problem here. You need a control group for comparison, to take into account normal fluctuations caused by such things as the season, eating a new diet provided by the investigators, and having a doctor poking at you. That control group was the group eating monounsaturated fat. The comparison between diet groups was the comparison that matters most, and it wasn't quite significant. I think the most you can say about this study is that it provides weak evidence that saturated fat decreases insulin sensitivity.
So we have five studies through 2008, which overall offer little support the idea that saturated fat reduces insulin sensitivity in non-diabetics. Since the review paper was published, I know of one subsequent study that asked the same question (3). Susan J. van Dijk and colleagues fed volunteers with abdominal overweight a diet rich in either saturated fat or monounsaturated fat. I e-mailed the senior author and she said the saturated fat diet was "mostly butter". After 8 weeks, insulin sensitivity was virtually identical between the two groups. This study appeared well controlled and used the gold standard method for assessing insulin sensitivity, called the euglycemic-hyperinsulinemic clamp technique***.
The evidence from controlled trials is rather consistent that saturated fat has no major effect on insulin sensitivity in humans, at least on time scales of a few months.
UPDATE: other trials have added to this finding. The large European LIPIGENE randomized controlled diet trial found that substantial differences in SFA intake had no effect on insulin sensitivity over 12 weeks in people with the metabolic syndrome (3b).
* For the nerds: euglycemic-hyperinsulinemic clamp (the gold standard), insulin suppression test, or intravenous glucose tolerance test with Minimal Model. They didn't include studies that reported HOMA as their only measure, because it's not very accurate.
*** They did find that markers of inflammation in fat tissue were higher after the saturated fat diet.
Lucy was a vegetarian and sapiens an omnivore: Plant foods as natural supplements
Early hominid ancestors like the Australopithecines (e.g., Lucy) were likely strict vegetarians. Meat consumption seems to have occurred at least occasionally among Homo habilis, with more widespread consumption among Homo erectus, and Homo sapiens (i.e., us).
The figure below (from: becominghuman.org; click on it to enlarge) shows a depiction of the human lineage, according to a widely accepted theory developed by Ian Tattersall. As you can see, Neanderthals are on a different branch, and are not believed to have been part of the human lineage.
Does the clear move toward increased meat consumption mean that a meat-only diet is optimal for you?
The answer is “perhaps”; especially if your ancestors were Inuit and you retained their genetic adaptations.
Food specialization tends to increase the chances of extinction of a species, because changes in the environment may lead to the elimination of a single food source, or a limited set of food sources. On a scale from highly specialized to omnivorous, evolution should generally favor adaptations toward the omnivorous end of the scale.
Meat, which naturally comes together with fat, has the advantage of being an energy-dense food. Given this advantage, it is possible that the human species evolved to be exclusively meat eaters, with consumption of plant foods being mostly optional. But this goes somewhat against what we know about evolution.
Consumption of plant matter AND meat – that is, being an omnivore – leads to certain digestive tract adaptations, which would not be present if they were not absolutely necessary. Those adaptations are too costly to be retained without a good reason.
The digestive tract of pure carnivores is usually shorter than that of omnivores. Growing a longer digestive tract and keeping it healthy during a lifetime is a costly proposition.
Let us assume that an ancient human group migrated to a geographical area that forced them to adhere to a particular type of diet, like the ancient Inuit. They would probably have evolved adaptations to that diet. This evolution would not have taken millions of years to occur; it might have taken place in as little as 396 years, if not less.
In spite of divergent adaptations that might have occurred relatively recently (i.e., in the last 100,000 years, after the emergence of our species), among the Inuit for instance, we likely have also species-wide adaptations that make an omnivorous diet generally optimal for most of us.
Meat appears to have many health-promoting and a few unhealthy properties. Plant foods have many health-promoting properties, and thus may act like “natural supplements” to a largely meat-based diet. As Biesalski (2002) put it as part of a discussion of meat and cancer:
“… meat consists of a few, not clearly defined cancer-promoting and a lot of cancer-protecting factors. The latter can be optimized by a diet containing fruit and vegetables, which contain hundreds of more or less proven bioactive constituents, many of them showing antioxidative and anticarcinogenic effects in vitro.”
Reference:
Biesalski, H.K. (2002). Meat and cancer: Meat as a component of a healthy diet. European Journal of Clinical Nutrition, 56(1), S2-S11.
The figure below (from: becominghuman.org; click on it to enlarge) shows a depiction of the human lineage, according to a widely accepted theory developed by Ian Tattersall. As you can see, Neanderthals are on a different branch, and are not believed to have been part of the human lineage.
Does the clear move toward increased meat consumption mean that a meat-only diet is optimal for you?
The answer is “perhaps”; especially if your ancestors were Inuit and you retained their genetic adaptations.
Food specialization tends to increase the chances of extinction of a species, because changes in the environment may lead to the elimination of a single food source, or a limited set of food sources. On a scale from highly specialized to omnivorous, evolution should generally favor adaptations toward the omnivorous end of the scale.
Meat, which naturally comes together with fat, has the advantage of being an energy-dense food. Given this advantage, it is possible that the human species evolved to be exclusively meat eaters, with consumption of plant foods being mostly optional. But this goes somewhat against what we know about evolution.
Consumption of plant matter AND meat – that is, being an omnivore – leads to certain digestive tract adaptations, which would not be present if they were not absolutely necessary. Those adaptations are too costly to be retained without a good reason.
The digestive tract of pure carnivores is usually shorter than that of omnivores. Growing a longer digestive tract and keeping it healthy during a lifetime is a costly proposition.
Let us assume that an ancient human group migrated to a geographical area that forced them to adhere to a particular type of diet, like the ancient Inuit. They would probably have evolved adaptations to that diet. This evolution would not have taken millions of years to occur; it might have taken place in as little as 396 years, if not less.
In spite of divergent adaptations that might have occurred relatively recently (i.e., in the last 100,000 years, after the emergence of our species), among the Inuit for instance, we likely have also species-wide adaptations that make an omnivorous diet generally optimal for most of us.
Meat appears to have many health-promoting and a few unhealthy properties. Plant foods have many health-promoting properties, and thus may act like “natural supplements” to a largely meat-based diet. As Biesalski (2002) put it as part of a discussion of meat and cancer:
“… meat consists of a few, not clearly defined cancer-promoting and a lot of cancer-protecting factors. The latter can be optimized by a diet containing fruit and vegetables, which contain hundreds of more or less proven bioactive constituents, many of them showing antioxidative and anticarcinogenic effects in vitro.”
Reference:
Biesalski, H.K. (2002). Meat and cancer: Meat as a component of a healthy diet. European Journal of Clinical Nutrition, 56(1), S2-S11.
Sunday, February 7, 2010
Thank You
I'd like to extend my sincere thanks to everyone who has supported me through donations this year. The money has allowed me to buy materials that I wouldn't otherwise have been able to afford, and I feel it has enriched the blog for everyone. Here are some of the books I've bought using donations. Some were quite expensive:
Food and western disease: health and nutrition from an evolutionary perspective. Staffan Lindeberg (just released!!)
Nutrition and disease. Edward Mellanby
Migration and health in a small society: the case of Tokelau. Edited by Albert F. Wessen
The saccharine disease. T. L. Cleave
Culture, ecology and dental anthropology. John R. Lukacs
Vitamin K in health and disease. John W. Suttie
Craniofacial development. Geoffrey H. Sperber
Western diseases: their emergence and prevention. Hugh C. Trowell and Denis P. Burkitt
The ultimate omega-3 diet. Evelyn Tribole
Our changing fare. John Yudkin and colleagues
Donations have also paid for many, many photocopies at the medical library. I'd also like to thank everyone who participates in the community by leaving comments, or by linking to my posts. I appreciate your encouragement, and also the learning opportunities.
Food and western disease: health and nutrition from an evolutionary perspective. Staffan Lindeberg (just released!!)
Nutrition and disease. Edward Mellanby
Migration and health in a small society: the case of Tokelau. Edited by Albert F. Wessen
The saccharine disease. T. L. Cleave
Culture, ecology and dental anthropology. John R. Lukacs
Vitamin K in health and disease. John W. Suttie
Craniofacial development. Geoffrey H. Sperber
Western diseases: their emergence and prevention. Hugh C. Trowell and Denis P. Burkitt
The ultimate omega-3 diet. Evelyn Tribole
Our changing fare. John Yudkin and colleagues
Donations have also paid for many, many photocopies at the medical library. I'd also like to thank everyone who participates in the community by leaving comments, or by linking to my posts. I appreciate your encouragement, and also the learning opportunities.
Saturday, February 6, 2010
Vitamin D levels: Sunlight, age, and toxicity
Calcidiol is a pre-hormone that is produced based on vitamin D3 in the liver. Blood concentration of calcidiol is considered to be a reliable indicator of vitamin D status. In the research literature, calcidiol is usually referred to as 25-Hydroxyvitamin or 25(OH)D. Calcidiol is converted in the kidneys into calcitriol, which is the active form of vitamin D.
The table below (from: Vieth, 1999; full reference at the end of this post; click on it to enlarge), shows the average blood vitamin D levels of people living or working in sun-rich environments. To convert from nmol/L to ng/mL, divide by 2.496. For example, 100 nmol/L = 100 / 2.496 ng/mL = 40.1 ng/mL. At the time of this writing, Vieth (1999) had 692 citations on Google Scholar, and probably more than that on Web of Science. This article has had, and continues having, a high impact among researchers.
The maximum average level of blood (or serum) vitamin D shown in the table is 163 nmol/L (65 ng/mL). Given that the human body produces vitamin D naturally from sunlight, it is reasonable to assume that those blood vitamin D levels are not yet at the toxic range. In fact, one of the individuals, a farmer in Puerto Rico, had a level of 225 nmol/L (90 ng/mL). That individual had no signs of toxicity.
Several studies show that pre-sunburn full-body exposure to sunlight is equivalent to an oral vitamin D intake of approximately 250 µg (10,000 IU).
In spite of claims to the contrary, vitamin D production based on sunlight does not cease after 40 years of age or so. Studies reviewed by Vieth suggest that among the elderly (i.e., those aged 65 or above) pre-sunburn full-body exposure to sunlight is equivalent to an oral vitamin D intake of 218 µg (8,700 IU).
Sunlight-induced vitamin D production does seem to decrease with age, but not dramatically.
Post-sunburn sunlight exposure does not increase vitamin D production. Since each person is different, a good rule of thumb to estimate the number of minutes of sunlight exposure needed to maximize vitamin D production is the number of minutes preceding sunburn. For a light-skinned person, this can be as little as 7 minutes.
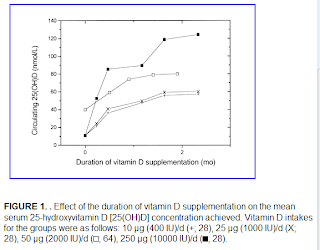
Vitamin D accumulation in the body follows a battery-like pattern, increasing and decreasing gradually. The figure below, from Vieth’s article, shows the gradual increase in blood vitamin D concentrations following the start of daily supplementation. This suggests that levels start to plateau at around 1 month, with higher levels reaching a plateau after 2 months.
While sunlight exposure does not lead to toxic levels of vitamin D, oral intake may. Below is a figure, also from Vieth’s article, that plots blood levels of vitamin D against oral intake amounts. The X’s indicate points at which intoxication symptoms were observed. While typically intoxication starts at the 50,000 IU intake level, one individual displayed signs of intoxication at 10,000 IU. That individual received a megadose that was supposed to provide vitamin D for an extended period of time.
Non-toxic levels of 10,000 IU are achieved naturally through sunlight exposure. This applies to modern humans and probably our Paleolithic ancestors. Yet, modern humans normally limit their sun exposure and intake of vitamin D to levels (400 IU) that are only effective to avoid osteomalacia, the softening of the bones due to poor mineralization.
Very likely the natural production of 10,000 IU based on sunlight was adaptive in our evolutionary past, and also necessary for good health today. This is consistent with the many reports of diseases associated with chronic vitamin D deficiency, even at levels that avoid osteomalacia. Among those diseases are: hypertension, tuberculosis, various types of cancer, gingivitis, multiple sclerosis, chronic inflammation, seasonal affective disorder, and premature senescence.
Reference:
Reinhold Vieth (May 1999). Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. American Journal of Clinical Nutrition, Vol. 69, No. 5, 842-856.
The table below (from: Vieth, 1999; full reference at the end of this post; click on it to enlarge), shows the average blood vitamin D levels of people living or working in sun-rich environments. To convert from nmol/L to ng/mL, divide by 2.496. For example, 100 nmol/L = 100 / 2.496 ng/mL = 40.1 ng/mL. At the time of this writing, Vieth (1999) had 692 citations on Google Scholar, and probably more than that on Web of Science. This article has had, and continues having, a high impact among researchers.
The maximum average level of blood (or serum) vitamin D shown in the table is 163 nmol/L (65 ng/mL). Given that the human body produces vitamin D naturally from sunlight, it is reasonable to assume that those blood vitamin D levels are not yet at the toxic range. In fact, one of the individuals, a farmer in Puerto Rico, had a level of 225 nmol/L (90 ng/mL). That individual had no signs of toxicity.
Several studies show that pre-sunburn full-body exposure to sunlight is equivalent to an oral vitamin D intake of approximately 250 µg (10,000 IU).
In spite of claims to the contrary, vitamin D production based on sunlight does not cease after 40 years of age or so. Studies reviewed by Vieth suggest that among the elderly (i.e., those aged 65 or above) pre-sunburn full-body exposure to sunlight is equivalent to an oral vitamin D intake of 218 µg (8,700 IU).
Sunlight-induced vitamin D production does seem to decrease with age, but not dramatically.
Post-sunburn sunlight exposure does not increase vitamin D production. Since each person is different, a good rule of thumb to estimate the number of minutes of sunlight exposure needed to maximize vitamin D production is the number of minutes preceding sunburn. For a light-skinned person, this can be as little as 7 minutes.
Vitamin D accumulation in the body follows a battery-like pattern, increasing and decreasing gradually. The figure below, from Vieth’s article, shows the gradual increase in blood vitamin D concentrations following the start of daily supplementation. This suggests that levels start to plateau at around 1 month, with higher levels reaching a plateau after 2 months.
While sunlight exposure does not lead to toxic levels of vitamin D, oral intake may. Below is a figure, also from Vieth’s article, that plots blood levels of vitamin D against oral intake amounts. The X’s indicate points at which intoxication symptoms were observed. While typically intoxication starts at the 50,000 IU intake level, one individual displayed signs of intoxication at 10,000 IU. That individual received a megadose that was supposed to provide vitamin D for an extended period of time.
Non-toxic levels of 10,000 IU are achieved naturally through sunlight exposure. This applies to modern humans and probably our Paleolithic ancestors. Yet, modern humans normally limit their sun exposure and intake of vitamin D to levels (400 IU) that are only effective to avoid osteomalacia, the softening of the bones due to poor mineralization.
Very likely the natural production of 10,000 IU based on sunlight was adaptive in our evolutionary past, and also necessary for good health today. This is consistent with the many reports of diseases associated with chronic vitamin D deficiency, even at levels that avoid osteomalacia. Among those diseases are: hypertension, tuberculosis, various types of cancer, gingivitis, multiple sclerosis, chronic inflammation, seasonal affective disorder, and premature senescence.
Reference:
Reinhold Vieth (May 1999). Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. American Journal of Clinical Nutrition, Vol. 69, No. 5, 842-856.
Thursday, February 4, 2010
How much vitamin D? Vitamin D Council's recommendations
Since my recent post on problems related to vitamin D deficiency and excess I received several questions. I have also participated in several discussions in other blogs related to vitamin D in the past few days.
There is a lot of consensus about vitamin D deficiency being a problem, but not much about vitamin D in excess being a problem as well.
Some bloggers recommend a lot of supplementation, which may be dangerous because: (a) our body evolved to obtain most of its vitamin D from a combination of sunlight exposure and cholesterol, and thus body accumulation regulation mechanisms are not designed to deal with excessive oral supplementation; and (b) vitamin D, like many fat-soluble vitamins, accumulates in fat tissue over time, and is not easily eliminated by the body when in excess.
The Vitamin D Council has the following general recommendation regarding supplementation:
There is a lot of consensus about vitamin D deficiency being a problem, but not much about vitamin D in excess being a problem as well.
Some bloggers recommend a lot of supplementation, which may be dangerous because: (a) our body evolved to obtain most of its vitamin D from a combination of sunlight exposure and cholesterol, and thus body accumulation regulation mechanisms are not designed to deal with excessive oral supplementation; and (b) vitamin D, like many fat-soluble vitamins, accumulates in fat tissue over time, and is not easily eliminated by the body when in excess.
The Vitamin D Council has the following general recommendation regarding supplementation:
Take an average of 5,000 IU a day, year-round, if you have some sun exposure. If you have little, or no, sun exposure you will need to take at least 5,000 IU per day. How much more depends on your latitude of residence, skin pigmentation, and body weight. Generally speaking, the further you live away from the equator, the darker your skin, and/or the more you weigh, the more you will have to take to maintain healthy blood levels.
They also provide a specific example:
For example, Dr. Cannell lives at latitude 32 degrees, weighs 220 pounds, and has fair skin. In the late fall and winter he takes 5,000 IU per day. In the early fall and spring he takes 2,000 IU per day. In the summer he regularly sunbathes for a few minutes most days and thus takes no vitamin D on those days in the summer.
For those who have problems with supplementation, here is what Dr. Cannell, President of the Vitamin D Council, has to say:
For people who have trouble with supplements, I recommend sunbathing during the warmer months and sun tanning parlors in the colder months. Yes, sun tanning parlors make vitamin D, the most is made by the older type beds. Another possibility is a Sperti vitamin D lamp.
One thing to bear in mind is that if your diet is rich in refined carbohydrates and sugars, you need to change that before you are able to properly manage your vitamin D levels. You need to remove refined carbohydrates and sugars from your diet. No more white bread, bagels, doughnuts, table sugar, sodas sweetened with high-fructose corn syrup; just to name a few of the main culprits.
In fact, a diet rich in refined carbohydrates and sugars, in and of itself, may be one of the reasons of a person''s vitamin D deficiency in the case of appropriate sunlight exposure or dietary intake, and even of excessive levels of vitamin D accumulating in the body in the case of heavy supplementation.
The hormonal responses induced by a diet rich in refined carbohydrates and sugars promote fat deposition and, at the same time, prevent fat degradation. That is, you tend to put on body fat easily, and you tend to have trouble burning that fat.
This causes a "hoarding" effect which leads to an increase in vitamin D stored in the body, and at the same time reduces the levels of vitamin D in circulation. This is because vitamin D is stored in body fat tissue, and has a long half-life, which means that it accumulates (as in a battery) and then slowly gets released into the bloodstream for use, as body fat is used as a source of energy.
It should not be a big surprise that vitamin D deficiency problems correlate strongly with problems associated with heavy consumption of refined carbohydrates and sugars. Both lead to symptoms that are eerily similar; several of which are the symptoms of the metabolic syndrome.
In fact, a diet rich in refined carbohydrates and sugars, in and of itself, may be one of the reasons of a person''s vitamin D deficiency in the case of appropriate sunlight exposure or dietary intake, and even of excessive levels of vitamin D accumulating in the body in the case of heavy supplementation.
The hormonal responses induced by a diet rich in refined carbohydrates and sugars promote fat deposition and, at the same time, prevent fat degradation. That is, you tend to put on body fat easily, and you tend to have trouble burning that fat.
This causes a "hoarding" effect which leads to an increase in vitamin D stored in the body, and at the same time reduces the levels of vitamin D in circulation. This is because vitamin D is stored in body fat tissue, and has a long half-life, which means that it accumulates (as in a battery) and then slowly gets released into the bloodstream for use, as body fat is used as a source of energy.
It should not be a big surprise that vitamin D deficiency problems correlate strongly with problems associated with heavy consumption of refined carbohydrates and sugars. Both lead to symptoms that are eerily similar; several of which are the symptoms of the metabolic syndrome.
Subscribe to:
Posts (Atom)